Rater Services, Reliability, and Scale Management
Errors in the administration and calculation of assessments can dramatically increase variability during a clinical trial, making it more difficult to detect signal in primary and secondary endpoints. The Clinical Assessment Technologies (CAT) group at Worldwide Clinical Trials is a fully integrated part of our study team that helps enhance signal detection by improving the quality of sites, the quality of patients, and the quality of the outcomes data.
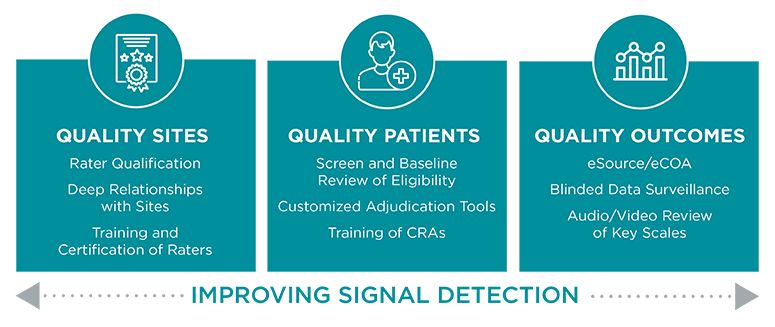
Key Benefits of Rater Services
As a comprehensive, deeply experienced, and integrated group within Worldwide, the CAT team provides the following benefits:
- Assurance that the best sites are selected based on CAT input regarding the qualifications of potential raters as well as previous experience regarding the quality of data from potential raters and sites
- Seamless communication across key team members in Study Startup, Clinical Operations, Project Management, Data Management and Medical Monitoring
- Elimination of external rater reliability vendors and the associated costs (time and monetary) associated with additional vendors
- Faster, more integrated site and study startup processes, with the ability to target specific sites for “FastTrack” activation 4-8 weeks before the Investigator Meeting
- CRAs who are trained by CAT provide a more specific and targeted review of key scale documents and enhanced monitoring of protocol and study deviations