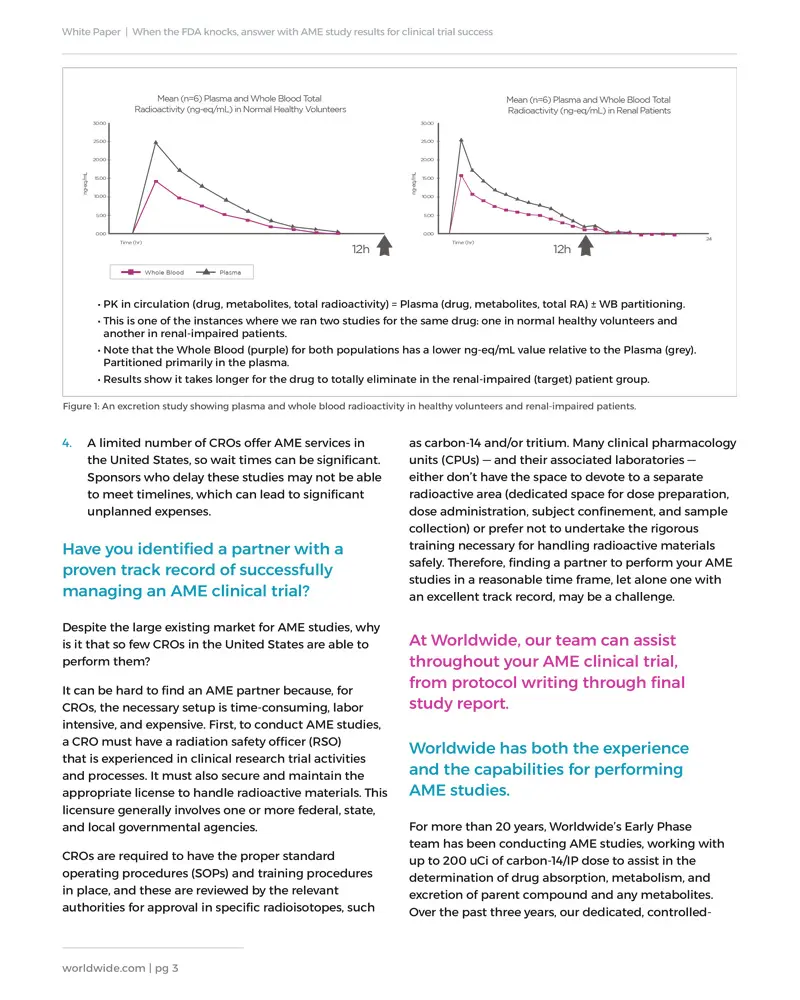
While AME studies traditionally have been conducted during Phase II clinical development or later, earlier investment in these activities — on the heels of preliminary studies, such as first-in-human, dose escalation, and food effect — often proves worthwhile. Furthermore, recently, the FDA has been requesting AME data sooner in the development process. Proactive AME studies will help lay the groundwork for the advanced phases of your study and provide valuable data to satisfy regulators as needed. Download our white paper to learn more.